Abstract
Introduction: Chimeric antigen receptor (CAR) T-Cell therapy is approved for relapse/refractory diffuse large B cell lymphoma (DLBCL) patients after two lines of therapy. Although it is an effective treatment, approximately 20-30% of patients have an early relapse. In this context, biomarkers that helps to identify those patients who will be refractory to this therapy are relevant. Cell-free DNA (cfDNA) has emerged as a new tool for non-invasive monitoring of patients with lymphoma, therefore the aim of this study was to evaluate dynamic cfDNA concentration in addition to other biomarkers (LDH, CRP, ferritin) before CAR T-cell infusion to detect early progressors.
Methods: We selected 44 r/r DLBCL patients treated with CAR T-cell in our centre. Plasma samples were collected pre-apheresis (PA) and pre-infusion (PI)(∆cfDNA). Other biomarkers (LDH, CRP, Ferritin) were studied PA, pre-lymphodepletion (PL) and PI, tumour volume metabolic (TMV) are also studied.
CfDNA were obtained from plasma using QIAamp® Circulating Nucleic Acid (Qiagen) and quantified by QuantiFluor dsDNA System (Promega). The Mann-Whitney test was used to compare differences between two independent quantitative variables . ROC analysis was conducted to determine the cut-off value of biomarkers. Cumulative incidence of progression (1 and 3 months) was calculated from the date of CAR T-cell infusion. Data analysis was performed using Stata.
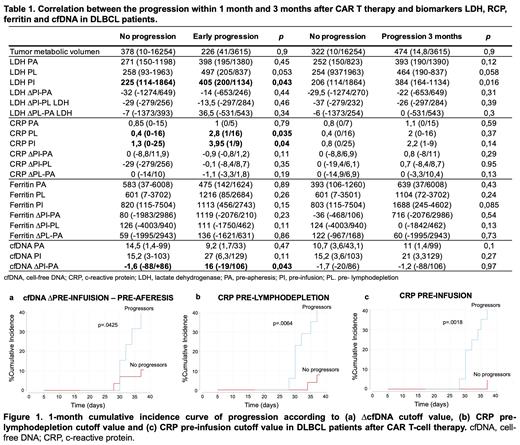
Results: Cumulative incidence of relapse at 1 month and 3 months was 20% and 30% respectively. The correlation between the progression (1 month, 3 months) and the different biomarkers is shown in Table 1. The CRP PL, CRP PI, LDH PI and ∆cfDNA (PI-PA, median time 41.5 days [range:31-107]) was correlated with early progression (1 month). No differences were found with progression at 3 months. The different cut-off for the biomarkers selected was 9 ng/mL for ∆cfDNA, 225 U/L for LDH and 1.35 mg/dL for CRP. Cumulative incidence of progression at 1 month was calculated for these biomarkers (figure 1): ∆cfDNA, HR: 4.3 (1.05-17), p=0.042; CRP PL: HR: 6.9 (1.5-31.1), p=0.012; CRP PI, HR: 11.2 (1.5-83), p=0.019 and LDH PI, HR: 3.4 (1-17) p=0.11. It should be noted that 83.3% (10/12) of the patients who progressed during the first month had at least one of the above variables.
Conclusions: Our results highlight that increase in cfDNA higher than 9 ng/mL, CRP PL and PI >1.35 mg/dL and LDH PI > 225 U/L before CAR T-cell therapy may detect early progressors within the first month after treatment. Therefore, ∆cfDNA, CRP and LDH could be useful to identify patients who highly probable will not benefit from the CAR T infusion, in which another prior strategy could be considered in an attempt to control the disease. These results need to be confirmed with a larger cohort.
Bailén: Pfizer: Honoraria; Kite-Gilead: Honoraria; Gilead: Honoraria. Kwon: Gilead: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal